Tissue architecture
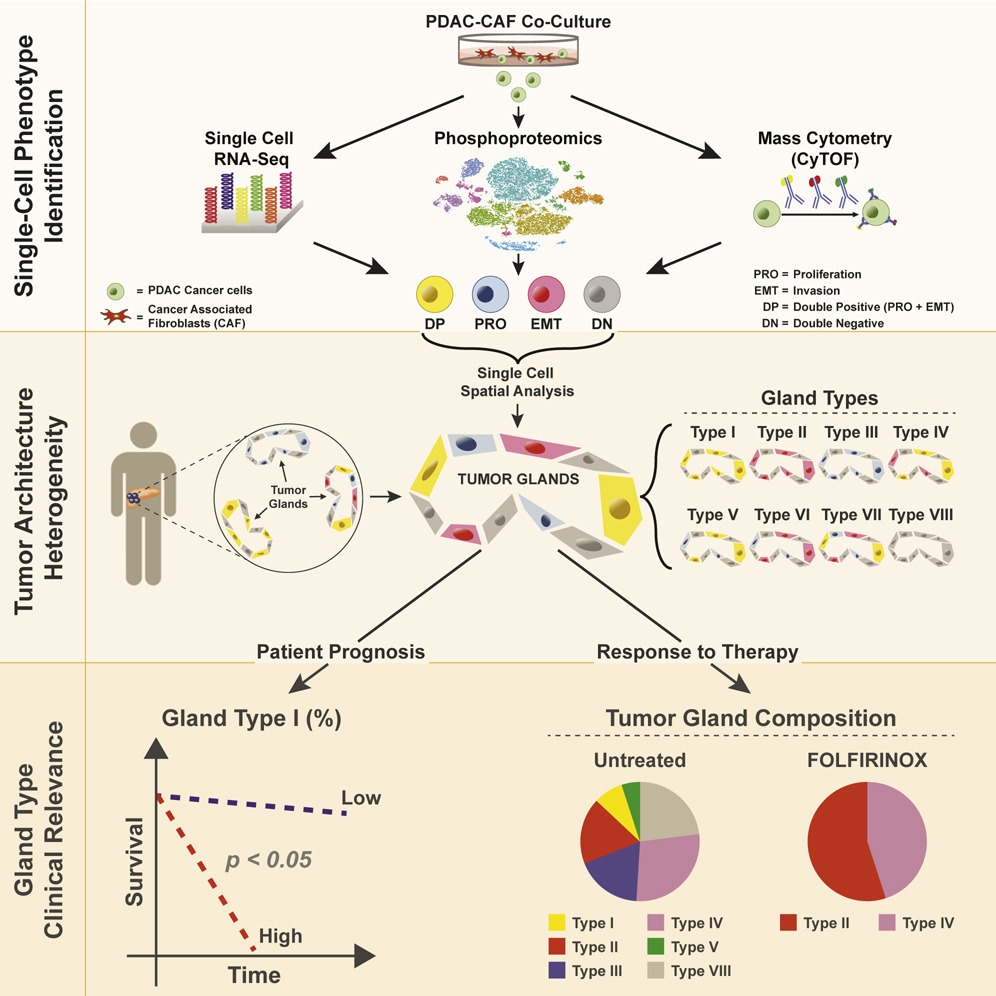
Example work: A major recent project involved defining structural and functional units of the tumor microenvironment in Pancreatic Ductal Adenocarcinoma (PDAC), the most common type of pancreatic cancer. The tumors are comprised of a complex microenvironment where non-cancer cells including fibroblasts and immune cells can make up >90% of the volume. Conflicting evidence as to whether cancer-associated fibroblasts (CAFs) support or hinder cancer cell aggressiveness suggests that a complex, context-dependent signaling relationship between these cell types. In a recent collaboration with David Ting’s lab at MGH we used an in-vitro single-cell RNA-seq approach to identify distinct cancer cell states that are induced through exposure to varying concentrations of CAFs including invasive EMT (epithelial -mesenchymal transition) and proliferating phenotypes. We then used RNA in-situ hybridization (RNA-ISH) to spatially map the locations of these cell states in 195 patient tumors, and characterized the tumor gland structures that they organize into. We found that typing spatially resolved tumor gland structures by the combination of individual cell types they comprise provided an additional lens into intra-tumoral heterogeneity, with correlations to stromal (CAF) content and clinical outcomes that were not discernible from single cell phenotypes alone. For example, we identified a cell state characterized by simultaneous activation of the proliferation and EMT gene expression programs. While the overall proportion of this cell state in a tumor (as could be characterized by scRNA-seq) was not predictive of outcomes, we found that the degree to which they were spatially clustered into glands as opposed to spread more evenly through the tumor mass was strongly associated with survival. We are now extending this work through development of analysis methods for newer spatial transcriptomics assays such as the Nanostring CosMx platform.